
Richard Baxter, MD – Board-Certified Plastic Surgeon
I have been working on the concept of an internal bra for more than 20 years now, and the idea has finally taken hold. But even as it becomes popular, there still seems to be some confusion about what it is and when to use it. In simple terms, an internal bra provides the same function as an external bra: to enhance the shape of the breast and provide support. In plastic surgery, this applies to breast lifts, breast reductions, and support of implants that have fallen out of position. Here, I will review when the internal bra is helpful, what materials are used, and practical advice.
Mesh Internal Bra with Breast Lifts and Reductions
Whether for support of the breast alone or for breast implants, the common denominator is a lack of adequate support by the patient’s own tissue. Breasts that need lifting have sagged because the skin and ligaments that support the breast have thinned, stretched, and weakened, which is why all versions of breast lifting lose fullness in the upper pole over time; they rely on the weakened tissue that caused the sagging in the first place. The same applies to breast reductions; even though the breast is being made smaller, there may still be insufficient strength of the tissues to maintain the shape over time, especially for dense breasts. This version of an internal bra uses a mesh implanted under the skin, just like an external bra but on the inside. By offloading the weight of the breast, there is less strain on the skin, creating a more favorable situation for healing and possibly better scarring.
Internal Bra for Implant Support
Breast implants also need support to maintain their position. Larger implants are more prone to sagging and more likely to benefit from adding support. Sometimes, the internal bra is placed at the same time as the implants if it is determined that there is a higher probability of sagging, but most often, it is done as part of a revision. In this instance, the bra is placed around the implant rather than under the skin. The material can be either mesh or an ADM (Acellular Dermal Matrix) such as Strattice.
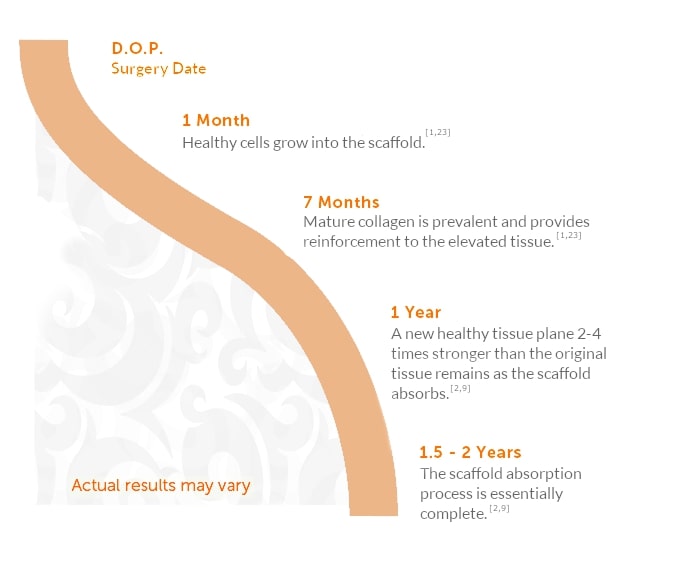
The mesh material used for most internal bra procedures is called Galaflex, a polymer made from a naturally occurring biomolecule called P4HB. I’ve tried different mesh materials over the years, but each of them had limitations. When I was invited to participate as an investigator in a clinical trial of Galaflex, I was eager to see whether the anticipated advantages would prove out. The trial did prove that the Galaflex internal bra helped to maintain upper pole fullness and very few problems. The advantages of the Galaflex internal bra include:
- Made from a natural, nontoxic, biocompatible material
- Slowly dissolves over about 15-18 months, leaving a layer of the patient’s own tissue
- Does not interfere with mammograms
- Feels natural
- Excellent safety record as a hernia mesh under a different brand name (Phasix)
For these reasons, Gala is still my go-to for breast lifts and reductions.
Breast implant support in reconstructive surgery after mastectomy is where the whole idea began. In fact, it could be said that the use of ADM revolutionized breast reconstruction. The first ADM was called Alloderm, and it is still widely used. I began using it in revisions of breast reconstruction cases where implant coverage and support were often lacking. It proved to be very useful, and I published a series of cases in 2003 including proof that it integrated and transformed into living tissue. Strattice, introduced a few years later, is made from pigskin processed to make it nearly identical to humans. Its primary role is in revision cosmetic implant cases.
A fortuitous finding from experience with ADM in breast reconstruction was that capsular contracture, a hardening of the tissue around breast implants, was much less common than reconstruction without. It is now used in treatment of recurrent capsular contracture, giving patients a very effective option to minimize the chances of it happening again.
FDA Disclaimer
Despite the record of safety and efficacy of these internal bra products and techniques, a recent statement from the FDA sought to clarify that they are not specifically approved for breasts. That doesn’t mean we can’t do it, but the companies that make them can’t promote them as internal bras. Their on-label use is “transitory soft tissue support.” This is the sort of reverse logic we have come to expect from the FDA in plastic surgery. We aren’t supposed to use them to support the breast, only soft tissue, as though the breasts aren’t soft tissue. Or to support implants in reconstruction when the breast has been removed by mastectomy; apparently, plastic surgeons have gotten so good that when we create something that looks like a breast, it is a breast as far as they are concerned. Good to know.
One mesh material, called Durasorb, is seeking FDA clearance for breast surgery. I have used Durasorb for tummy tucks (abdominoplasty) where there was a wide muscle separation and weak fascia, and it works well. However, it dissolves much faster than Gala, so I have been hesitant to use it for this purpose. Other materials have been tried, but they have yet to catch on.
Our expertise, coupled with science-based technologies and the latest in advanced techniques at PHASE Plastic Surgery, is how we continue to be the best surgical and non-surgical facility Seattle has to offer. We’re honored and excited to join you on your aesthetic journey toward looking and feeling your best. To get started, complete our consultation form, or call us at (425) 776-0880.



